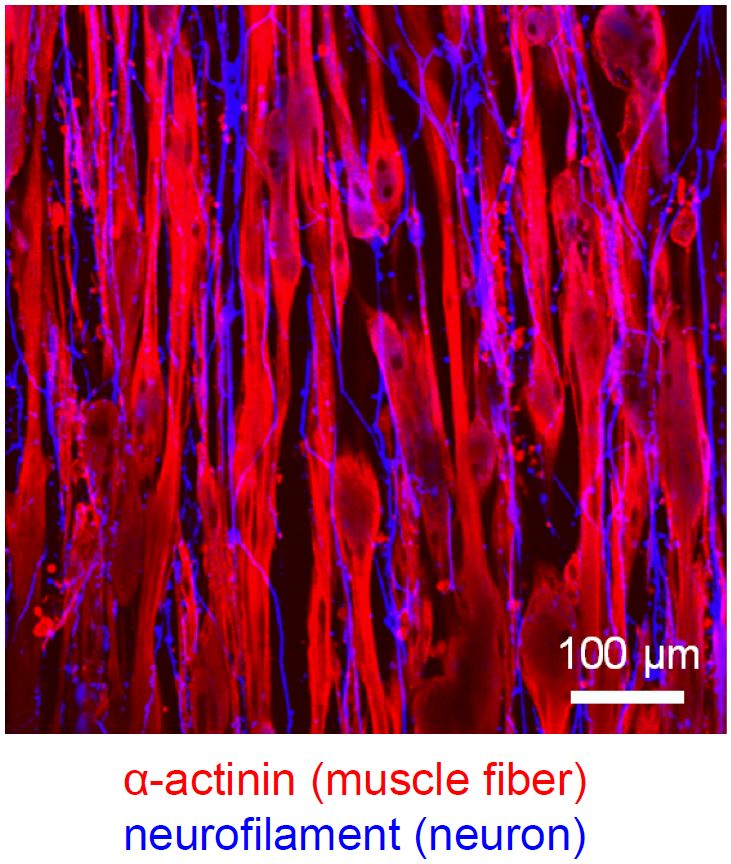
Approach for neuron-muscle tissue engineering
Since skeletal muscle tissues in the body function through the signaling from nerve system, disorders of neurons often influence the abilities of muscle tissues. Therefore, when we attempt to develop a new tissue engineering approach to treat muscle diseases, the interactions between neurons and muscles need to be considered. We previously developed a method to engineer human muscle tissue constructs. Based on this tissue engineering method, we now collaborate with Takeda Lab in Waseda University to produce neuron-muscle tissue constructs. To date, we succeeded in formation of native-like aligned neuron networks by co-culturing human iPS-derived neurons within the muscle tissue construct. In addition, we confirmed that acetylcholine receptor receiving the signals from neurons significantly generated on the engineered myofibers. We expect that in the future this approach can be used to develop new treatment methods for neuron-muscle diseases.
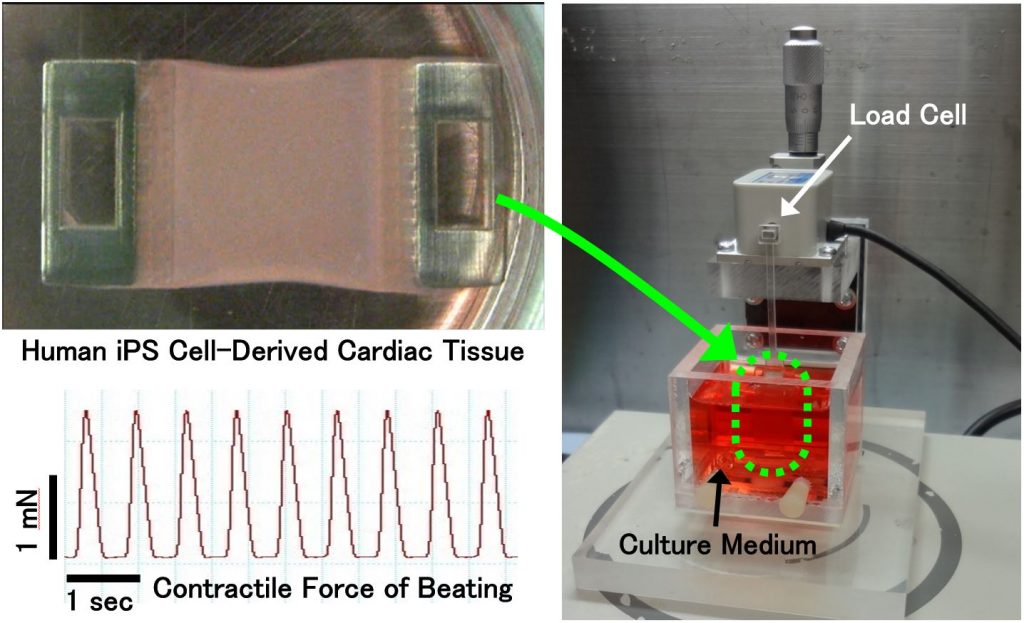
Contractile force measurement of engineered cardiac tissue
Recently, the technologies of preparing cardiomyocytes from human iPS cells have been advanced. Consequently, human iPS cell-derived cardiomyocytes are commercialized by several companies at present. These cardiomyocytes are used for the researches of cardiac regenerative medicine. Concurrently, the non-clinical safety pharmacology studies using these cardiomyocytes are also proceeding globally. In the present study, we developed the experimental system to measure the contractile force of engineered cardiac tissues fabricated from human iPSC-derived cardiomyocytes1). The effectiveness of the system for in vitro drug efficacy and cardiotoxicity testing is currently being demonstrated. Besides, the commercialization of the system is also proceeding by the collaboration with Nihon Kohden Corporation. We aim for the establishment of cardiac non-clinical testing methods that partly substitute for the present animal experiments and clinical trials in new drug development. Additionally, the system is also being used for the functional evaluation of engineered cardiac tissues aimed for transplantation, and for the pathological studies by measuring the contractility of cardiac diseased tissue models.
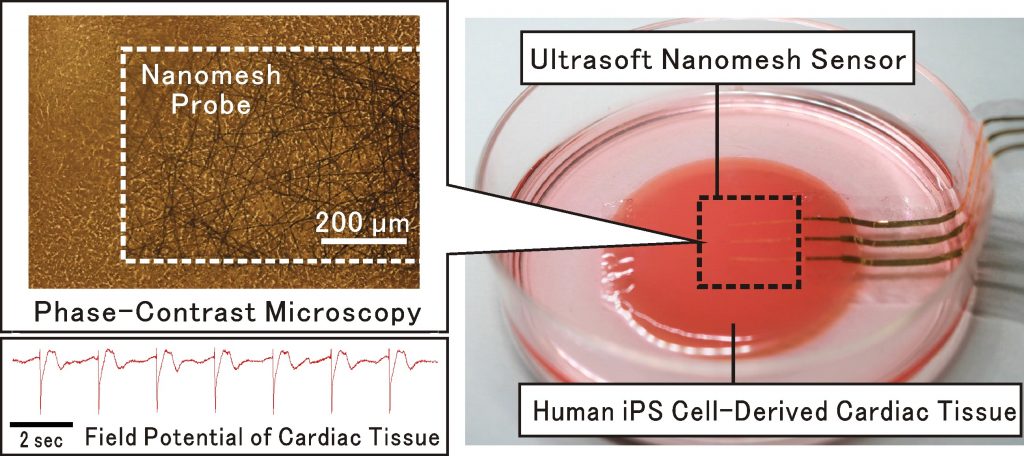
Ultrasoft nanomesh sensor for monitoring electrical potential of engineered cardiac tissues
Recent technological developments on stem cell differentiation and tissue engineering enable the fabrication of human cardiac tissues that can beat dynamically in vitro, like real heart. In this research, collaborating with Prof. T. Someya in Tokyo University, we have developed the ultrasoft nanomesh sensor to monitor electrical field potential of beating cardiac tissues prepared from human iPS cells, without any inhibition of beating movement2). This technology is valuable to achieve in-vitro drug safety and efficacy testing that can replace the animal experiments, and to evaluate the contractile properties of engineered cardiac tissues for regenerative therapies. Furthermore, it is hopeful that this technology will contribute to the development of electronics-fused advanced-functional tissues/organs, and innovative biological actuators.
[1]Lee et al. Nat Nanotechnol. 2018; 14: 156-160
Understanding the underlying mechanisms of heart failure using human iPS cell-derived tissue models
Heart failure is end stage of various types of heart disease and the current situation is called as “Heart failure pandemic” due to the increasing number of patient annually. However, the mechanisms have been fully elusive and the novel therapeutic strategies for the disease onset and disease progression are greatly expected. Based on our fundamental technologies including iPS cell-derived cardiovascular cell mass production and cell sheet engineering, we have succeeded to fabricate various types of heart failure tissue models using iPS cells from patients with hereditary dilated cardiomyopathy and hypertrophic cardiomyopathy, anti-cancer drugs and hypoxic environment. Understanding the underlying mechanisms using these models with molecular biology technologies will provide us the new insights of key pathways for the drug development.