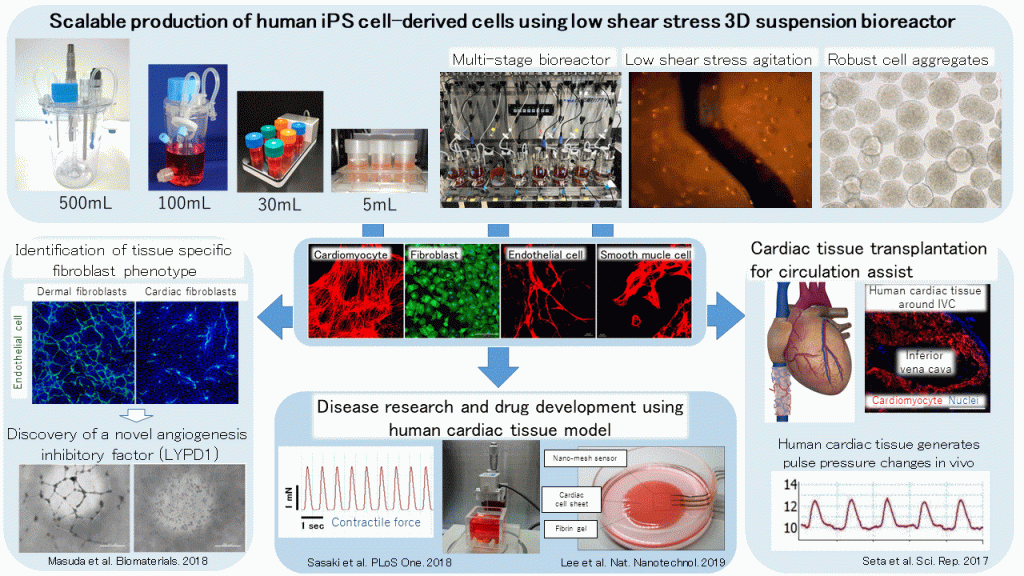
Development of bioengineered human cardiac tissue through the development of scalable culture system of human iPS cells for regenerative medicine, and disease and drug discovery
Human pluripotent stem cells (hPSCs) including human induced pluripotent stem cells (hiPSCs) have been applied worldwide as promising cell sources for regenerative medicine, disease research and drug development. However, stable and scalable mass production technology for target cells is indispensable for practical use and acceleration of research and development. We recently have developed the novel 3D suspension bioreactor system with the low shear stress agitation technology, which enables the high density culture of hiPSCs-derived cell aggregates. The development of various sizes of bioreactor including a small scale bioreactor for culture condition optimization to a practical litter-scale bioreactor is capable of seamlessly scaling up the cell manufacturing process, through a medium exchange system, culture environment monitoring technology development, and cell-processing system development after mass production. Nowadays, these developing technologies strongly promote the practical application of regenerative medicine, drug discovery and disease research using hPSCs all over the world. Notwithstanding the hardware development, we have also developed a wide varieties of 3D mass culture strategies for undifferentiated hiPSCs expansion and the differentiation to cardiomyocytes, vascular endothelial cells, fibroblasts, pancreatic endocrine/exocrine cells and thyroid follicular cells.
Disease and drug discovery research is mainly conducted using animal models, but from the viewpoint of species differences, there is high expectation for human cells. Since it is controlled by the mutual interaction of various cells in tissues and organs, it is desirable to develop a tissue model that is closer to the living body. We are constructing a cardiac tissue model using cell sheet engineering and mass production technology of hiPSCs-derived cardiovascular cells. In addition to the electrophysiological evaluation of cardiac tissue, we have developed various types of physiological evaluation systems for tension measurement of single-layer cardiac cell sheet and pulse pressure measurement of tubular cardiac tissue. These technologies can be applied to not only drug discovery research through the evaluation of drug cardiotoxicity, but also the elucidation of the molecular mechanisms of hereditary cardiomyopathy and various heart failure pathologies, and the elucidation of the maturation mechanism of cardiac tissue. We also expect that understanding the molecular mechanisms of mutual cellular interactions in cell sheets will lead to clarify the underlying mechanisms of the homeostasis of living tissues and organs. Recently, we have identified the ability of cardiac fibroblasts to suppress angiogenesis and its responsible factor LYPD1, and elucidated the novel mechanism of maintaining cardiac homeostasis via angiogenesis-inhibiting system using various molecular biological techniques. We are also working on the development of angiogenesis treatment strategies through inhibiting LYPD1.